Biochemical Routes for Production of Advanced Biofuels
Several technological routes for the production of biofuels from lignocellulosic materials are based on biochemical processes, i.e., conversion processes that use microorganisms or enzymes as catalysts.
Enzymatic processes can be used for the fractionation and solubilization of lignocellulosic biomass, for example, in the enzymatic hydrolysis of the cellulose and hemicellulose fractions of biomass, generating simple sugars such as glucose and xylose. The sugars present in these hydrolysates can then be used in fermentative processes to produce biofuels considered “advanced” or “second generation” because of their lignocellulosic origin. Typically, the same biofuels traditionally obtained from sugar or starch raw materials can be produced in this way, such as ethanol and butanol produced from sugarcane juice or corn starch, which for this reason are considered “conventional” or “first-generation”.
The most commonly used fermentation processes for the production of biofuels are alcoholic fermentation, which uses yeast of the genus Saccharomyces to produce ethanol from some simple sugars, and butanol fermentation, in which bacteria of the genus Clostridium convert simple sugars into a mixture of butanol, ethanol and acetone. Both ethanol and butanol are biofuels approved in many countries for automotive use as a substitute for gasoline. Also worth mentioning is anaerobic digestion, a process in which a consortium of microorganisms is used to convert a wide range of organic molecules (including polymerized ones) into biogas. Biogas is a mixture of methane and carbon dioxide that can be used directly as fuel in internal combustion engines, or purified by removing the CO2 to generate biomethane, a substitute for natural gas.
All the biochemical processes mentioned above take place under anoxic conditions, that is, in the absence of atmospheric oxygen. In the absence of a strong oxidizing agent such as oxygen, the ability of microorganisms to extract chemical energy from the substrates present is limited. As a result, a large part of the energy contained in the substrates remains present in the fermentation products. For example, in alcoholic fermentation, the ethanol produced still contains about 92% of the energy contained in glucose, although it has approximately half the original mass:
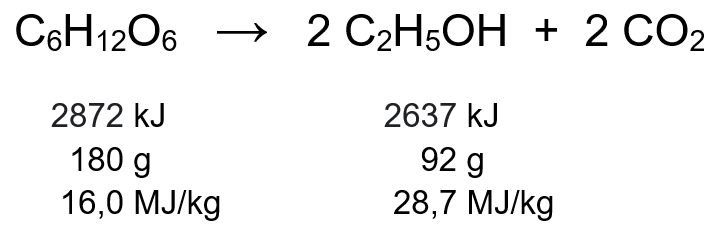
As a result, the biofuel obtained contains more energy per unit mass than the starting lignocellulosic material or the carbohydrate derived from it. This “energy-densification” effect is present in all the fermentative processes mentioned, and is due to a redistribution of the oxygen atoms contained in the substrate: most of them are concentrated in the CO2 produced in the fermentation, thus leaving the other product with an oxygen content lower than that of the carbohydrate used as feedstock. The lower the oxygen content in the produced biofuel, the higher its calorific value.
The enzymatic or microbial processes referred to above take place at temperatures of 30 to 50oC and at atmospheric pressure, conditions that are much milder than those used in thermochemical biomass conversion processes such as pyrolysis or gasification. As a result, the reactors used in industrial biochemical conversions are typically much simpler than the thermochemical reactors.
However, biochemical conversions are slower and take place in relatively dilute aqueous solutions, so they have lower volumetric yields compared to thermochemical conversions. When they require adjustment of the pH of the fermentation medium, biochemical processes can require significant amounts of acids or alkalis as operating inputs. Processing in aqueous media also entails a significant consumption of thermal energy in operations of concentration of sugar solutions fed to the bioreactors and/or separation of products by distillation, generating, in addition, important volumes of aqueous effluents (stillage) that may require treatment to remove their organic load before final disposal.
As a consequence, biorefineries that produce first-generation ethanol (1G ethanol) have a heat and power cogeneration unit sized to provide the plant with the required heat load, and may generate exportable surpluses of electricity. At the same time, the stillage from the distillery can be directed to an anaerobic digestion unit, allowing energy recovery from these diluted streams in the form of biogas.
Worldwide, the production of advanced biofuels by fermentation is incipient. However, important volumes of ethanol are produced by first-generation biochemical processes, mainly in the US (from corn starch) and Brazil (from sugarcane juice or molasses). In the second case, the production of first-generation ethanol (1G ethanol) is accompanied by the production of large surpluses of lignocellulosic residues in the form of bagasse and sugarcane straw, which could, for example, be destined for the fermentative production of second-generation ethanol (2G ethanol), in what would then be a 1G2G sugarcane biorefinery. Alternatively, part of the lignocellulosic residues can be used in thermochemical conversion processes integrated to the 1G biorefinery, providing opportunities to increase the energy efficiency of the entire process.
Finally, it should be noted that the alcoholic and butanolic fermentations and also obtaining biomethane from biogas are processes that originate very pure biogenic CO2 streams, which can be used in processes of geological carbon sequestration or production of synthetic biofuels using electrolytic hydrogen (“Power-to-X” processes), generating, in both cases, additional reductions in emissions of greenhouse gases.
Fundamental Bibliographical References
